PHAGE THERAPY,
RE-ENVISIONED.
We bio-hack bacterial resistance in skin disorders, through genetic engineering of phage endolysins.
About us
Asterion is a privately held biopharmaceutical dedicated to provide innovative solutions for bacterial resistance in inflammatory skin disorders.
In early 2018, the multi-disciplinary team at Asterion began to map out a scientific strategy, collaborations and operational plan. Each member brought a unique perspective and skill set to the internal collaboration.
Many other colleagues and partners; from business development to human resources to legal to finance; also contributed time and perspective, highlighting our focus on cross-functional partnership that is a hallmark of our culture.

for decades We have been using antibiotics as if there was no biological cost. hence, the massive problem we now have to face.
our purpose
The accelerating antibiotics crisis requires decisive action. Antibiotics frequently fail and destroy our natural skin microbiomes, and ultimately also change other microbiomes like the gut.
Our engineered recombinant endolysins derived from bacteriophages work where antibiotics fail and protect our beneficial bacteria. They are our best bet to defuse this existential crisis for modern medicine.
alexander fleming's prediction became reality
phages and their endolysins in nature
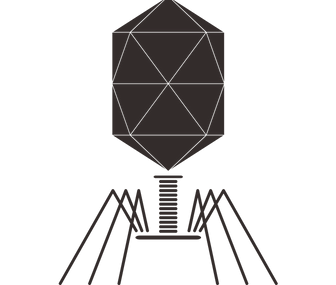
phages and their endolysins in nature
Phages are the most abundant biological entity on earth. Approximately 10³¹ populate our planet and they outnumber bacteria 10:1. Phages hunt, infect and kill bacteria and do this strain specific, and extremely precise.
Phages are bactericidal by nature and have the potential to be highly innovative novel anti-bacterials that work where antibiotics fail. Their precision allows the precise targeting of individual bacterial strains within the beneficial skin microbiome.
Many phages also encode a protein called endolysin. The phage needs the endolysin in the last step of its replication cycle to cleave the bacterial cell wall, ultimately killing the bacteria. Endolysins can also cleave the cell wall from the outside. This makes them a novel class of anti-bacterials with the potential to revolutionize the treatment of bacterial infections.
phages and their endolysin activity
phage infection
Phages hunt strain-specific bacteria to infect and use their host to replicate.

phage assembly

endolysin assembly

cell destruction
meet phagendo
Since the rise in multi-drug resistant skin infections seems unstoppable, alternative strategies to combat these pathogens are of high unmet need. Phagendo, a combination of the prefix “phage” (a virus that infects and replicates within bacteria) with an “endolysin” (the enzyme that a phage produces to kill a bacteria) is a novel antimicrobial platform technology to combat bacterial pathogens in a wide range of skin related disorders. Our platform can be adapted to design specific fusion endolysins that either target gram-positive or gram-negative bacterial species. Even multi-drug resistant or persistent cells can be addressed by their unique mode of action.
In addition, Phagendo’s have also been shown to be more effective in killing strain-specific bacteria in comparison to wild-type endolysins. This is due to the fusion of highly effective target peptide moieties, resulting in novel chimeric endolytic enzymes with improved affinity to the bacterial cell wall, (thermo)stability, and without the possibility of building up resistance.
overview
Phagendo’s are muralytic enzymes that:
- exhibit a new antimicrobial mechanism.
- have an adjustable specificity.
- target Gram-positive and Gram-negative bacteria.
- free of potential resistance being evoked.
- eliminate persistent cells.
- are active within biofilm.
- are quickly biodegradable.
- are non-toxic and do not cause negative side effects.
- are accepted by leading global companies.
phagendo's vs. antibiotics
While classic antibiotics require non-resistant cells, work within hours or even days and only kill cells that are metabolically active, our fast-acting Phagendo’s destroy sensitive, resistant and persistent bacterial cells within minutes.
Phagendo’s selectively attack the cell wall of gram-negative or gram-positive bacterial cells leading to cell death caused by high osmotic pressure. Antibiotics also target the bacterial cell wall. However, they inhibit the assembly of a functional peptidoglycan by specific binding to enzymes of dividing cells. They are translocated through the outer membrane via porins into the periplasmic space of the bacteria.
Subsequently, most antibiotics bind to enzymes called peptidoglycan transpeptidases and thus inhibit the crosslinking of peptidoglycan precursors to build the functional cell wall of metabolically active and dividing cells. This leads to the death of the bacteria.
Unlike antibiotics, Phagendo’s do not require an active metabolism because they work directly on the cell envelope by combining electrostatic and hydrophobic peptides with a muralytic moiety. Under high osmotic pressure, the inner membrane blebs out and finally the bacterial cell bursts.
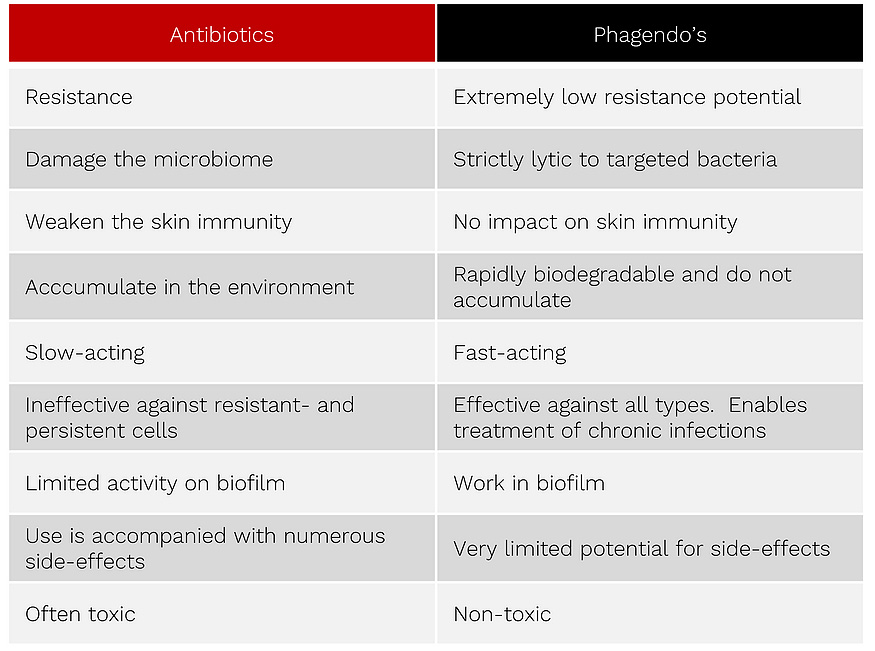

any serious technology should overcome resistance, persistence, and microbiome impairment.
sustainably produced

Phagendo’s are produced by turning plants into mini bio-factories. Plants are highly efficient at producing proteins of varying complexity, serving as mini bioreactors to effectively produce our antibacterials.
Our plant-based production platform demonstrates better agility, accuracy, and speed compared with other manufacturing technologies by eliminating the risk of pathogens and contamination during production, and significantly shortening production timelines.
Our platform scalability is quite simple; one plant or 10,000 plants require the same growth conditions whereas the capacity is adjustable to market needs.
when we mean fast, we mean
fast
from gene synthesis to fully functional
purified protein in as little as
13 days
production flow
SYNTHETIC GENE CLONING
1-2 days

clone into agrobacterium
2 days
protein extraction
5-6 days

agro-infiltration
1 day
protein extraction
1 day

isolation and purification
1 day
our purpose
The accelerating antibiotics crisis requires decisive action. Antibiotics frequently fail and destroy our natural skin microbiomes, and ultimately also change other microbiomes like the gut.
Our engineered recombinant endolysins derived from bacteriophages work where antibiotics fail and protect our beneficial bacteria. They are our best bet to defuse this existential crisis for modern medicine.
our deep & diverse pipeline
indication
preclinical
clinical + safety
regulatory
MRSA
(Methicillin-resistant Staphylococcus aureus)
Erythrasma
(Corynebacterium minutissimum)
Bacterial Vaginosis
(Gardnerella vaginalis)
Impetigo (combination treatment)
(Streptococcus and Staphylococcus aureus)
Eczema (combination treatment)
(Streptococcus and Staphylococcus aureus)
Rosacea (combination treatment)
(Bacillus oleronius)

Acne Vulgaris (combination treatment)
(Cutibacterium acnes)

CONTACT us
Please submit any questions, suggestions, or general feedback in the space below along with your name and email address.
